How to Find Number of Protons
The atomic number from the atomic mass will give you the calculated number of neutrons in the atom. There are 275 isotopes of the 81 stable elements in addition to over 800 radioactive isotopes and every element has known isotopic forms.
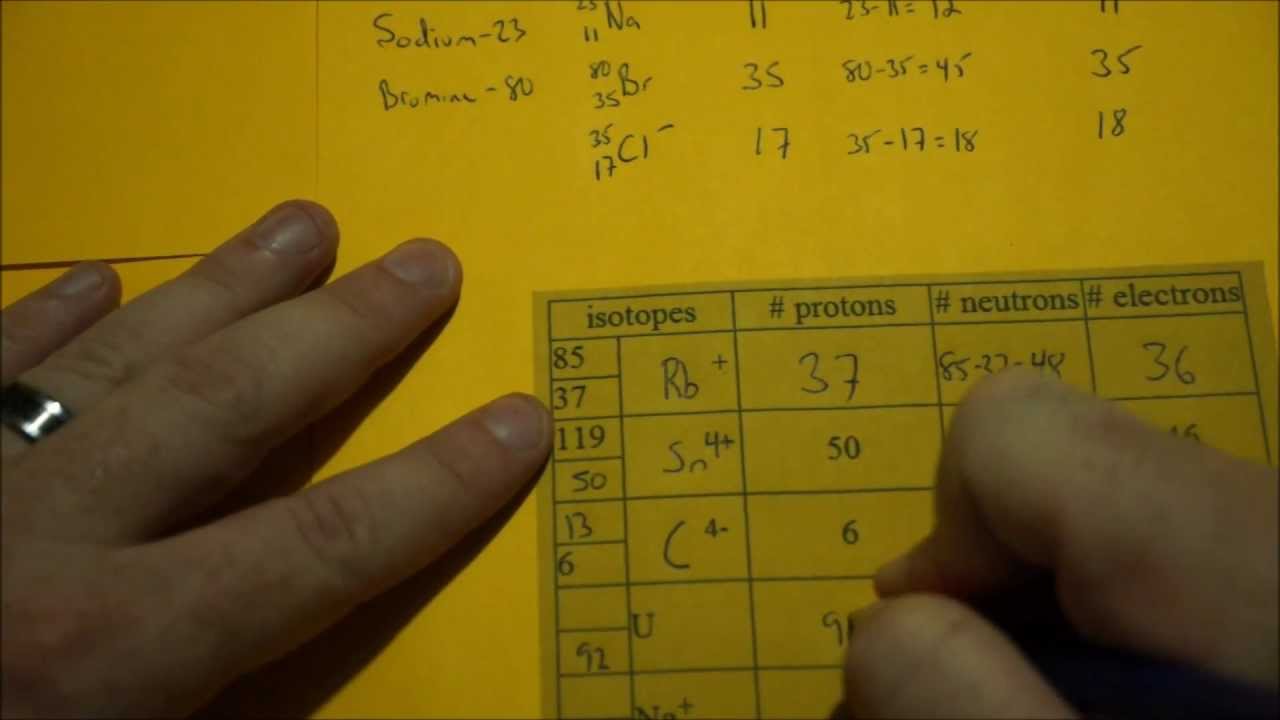
Determining Protons Neutrons And Electrons Atoms And Ions Protons Neutrons Electrons
How to easily find the number of electrons protons and neutrons in an oxygen atom.

. While other numbers may be decimal values the atomic number is always a simple positive whole number. You can find the atomic number from an isotope symbol. Of protons 17 of neutrons 37 17 20 of electrons 17 0 17 of protons 16 the atomic number is not given but can be found on the.
Quarks which are always bound within larger. Remember that the atomic number is the same as the number of protons which you have already identified. Each element is defined by the number of protons found in each of its atoms.
The atomic number of an element is found through the number of protons present in the nucleus. How to find the Atomic Mass. For our boron example 11 atomic mass 5 atomic number 6 neutrons.
Every nucleus of a. Number of protons 11. It is defined as where n q is the number of quarks and n q is the number of antiquarks.
Number of protons present in an atom. How to easily find the number of electrons protons and neutrons in a lithium atom. Scientist Henry Gwynn Jefferies Mosle examined the X-ray spectrum of various elements in 1913-to 1914.
Of protons atomic number of neutrons mass number atomic number of electrons atomic number charge. The atomic number of an element is simply the number of protons in. Protons make up most of the visible universe.
Carbon has 6 protons in its nucleus making it also the sixth element in the periodic table. Find the Number of Protons. Subtract the atomic number from the atomic mass.
Electron charge symbol e fundamental physical constant expressing the naturally occurring unit of electric charge equal to 1602176634 1019 coulomb. In particle physics the baryon number is a strictly conserved additive quantum number of a system. In fact its actually possible to have an atom consisting of only a proton ionized hydrogen.
It is the average weight of an atom or molecule. Great lets apply the rules to some examples. Lets also look at the next two alkanes propane and butane before trying to find some patterns for determining the number of NMR signals a little easier.
The results of his experiments show that each element has a unique integer equal to the number of positive charges in the nucleus of that element. No matter how many electrons or neutrons an atom has the element is defined by its number of protons. Scientist Henry Gwynn Jefferies Mosle examined the X-ray spectrum of various elements in 1913-to 1914.
A weighted average of the number of neutrons and protons present for all isotopes. Since the vast majority of an atoms mass is made up of its protons and neutrons subtracting the number of protons ie. For example if you are told the element name is aluminum you can find the name or symbol Al to determine the atomic number is 13.
Isotope definition any of two or more forms of a chemical element having the same number of protons in the nucleus or the same atomic number but having different numbers of neutrons in the nucleus or different atomic weights. FlexBook Platform FlexBook FlexLet and FlexCard are registered trademarks of CK-12 Foundation. Subtract the atomic number from the atomic mass.
The results of his experiments show that each element has a unique integer equal to the number of positive charges in the nucleus of that element. Protons together with electrically neutral particles called neutrons make up all atomic nuclei except for the hydrogen nucleus which consists of a single proton. The atomic number of a sodium atom is 11 and its mass number is 23.
To find the number of neutrons you will need to subtract the atomic number from the atomic mass. Calculate the number of protons neutrons and electrons it contains. Now in a new study published in the August 18 issue of the journal Nature scientists find that because of the strange nature of quantum physics.
He used all his force in opening the window. A pure substance that cannot be broken down into a simpler substance by chemical means. Force definition physical power or strength possessed by a living being.
Propane and butane give two signalsOne because the protons of the CH 2 group are different from those in the CH 3 group and the other because despite having four carbon atoms the molecule is a combination of two identical CH. Proton stable subatomic particle that has a positive charge equal in magnitude to a unit of electron charge and a rest mass of 167262 1027 kg which is 1836 times the mass of an electron. In cosmology recombination refers to the epoch during which charged electrons and protons first became bound to form electrically neutral hydrogen atomsRecombination occurred about 370000 years after the Big Bang at a redshift of z 1100The word recombination is misleading since the Big Bang theory doesnt posit that protons and electrons had been.
Build an Atom - PhET. The numbers after the decimal point represent the usually very small mass of the. Baryons three quarks have a baryon number of 1 mesons one quark one antiquark have a baryon number of 0 and antibaryons three antiquarks have a baryon.
Atomic mass is related to the number of neutrons and protons present in a particular nucleus of an element. The easiest way to find the atomic mass of an element is to look on the periodic table. In addition to the electron all freely existing charged subatomic particles thus far discovered have an electric charge equal to this value or some whole-number multiple of it.
The atomic number is usually the number of protons present in the nucleus of an element that you can also determine using this best atomic number calculator. How to find the Atomic Number. The atomic numbers go in order on the table.

Finding Protons Neutrons And Electrons Through The Atomic Number And Neutrons By Mass Atomic

3 Ways To Calculate Atomic Mass Wikihow Teaching Chemistry Atoms And Molecules For Kids Chemistry Worksheets
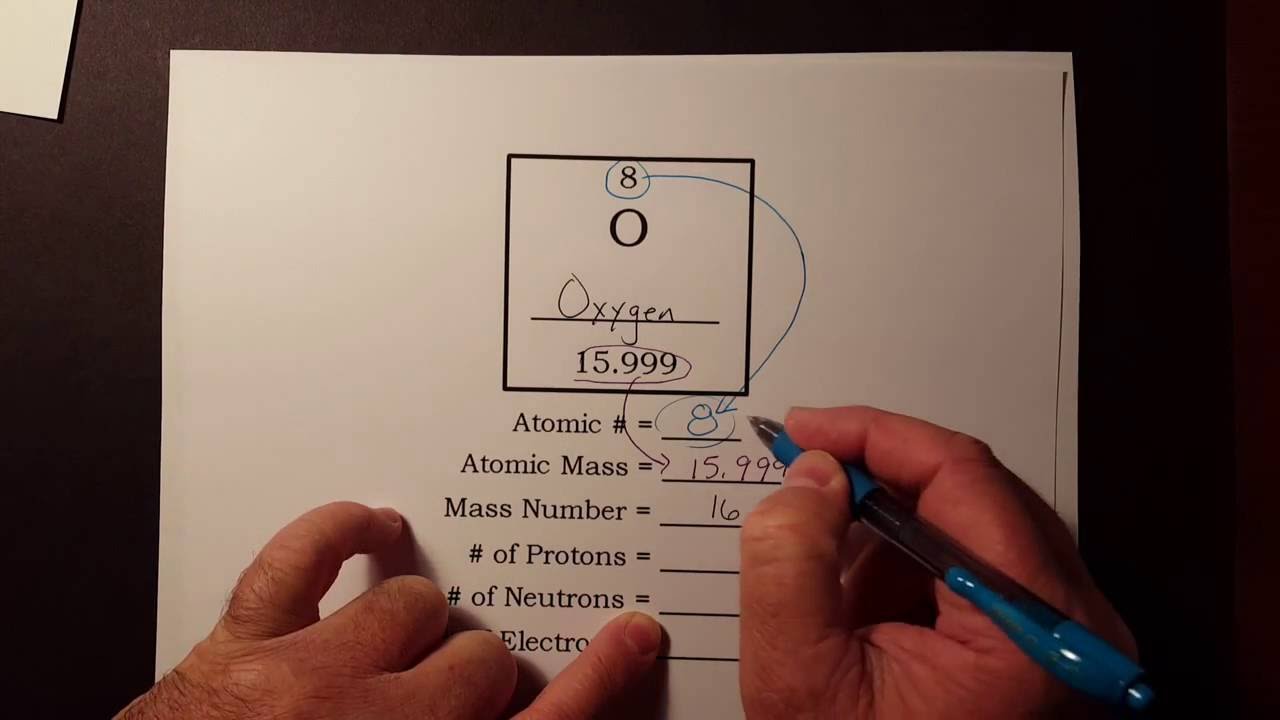
How To Find The Number Of Protons Neutrons And Electrons From The Periodic Table Youtube Neutrons Proton Neutron Electron Protons

No comments for "How to Find Number of Protons"
Post a Comment